MAGEMinApp
Effortlessly compute phase diagrams and Pressure-Temperature paths
A set of tools to compute phase equilibria for Earth-like compositions
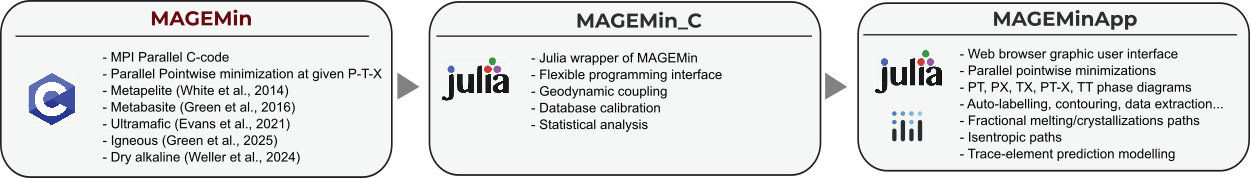
MAGEMin is a Gibbs energy minimization solver, which computes the thermodynamically most stable assemblage for a given thermodynamic database and a set of bulk-rock composition, pressure and temperature conditions. It returns the fraction of the stable minerals, their compositions and all thermodynamically-derived parameters such as density, thermal expansivity, heat capacity etc.
MAGEMin backend is written as a parallel C library and uses a combination of linear programming, the extended Partitioning Gibbs free Energy approach and gradient-based local minimization to compute the most stable mineral assemblage. In this, it differs from existing approaches which makes it particularly suitable to utilize modern multicore processors.
While MAGEMin is the engine for the prediction of the stable phases, it is best used wih the Julia interface MAGEMin_C and/or the web-browser julia MAGEMinApp.
Note
Please keep in mind that the datasets are only calibrated for a limited range of P,T and bulk rock conditions. If you go too far outside those ranges, MAGEMin (or most other thermodynamic software packages for that matter) may not converge or give bogus results. Developing new, more widely applicable, thermodynamic datasets is a huge research topic, which will require funding to develop the models themselves, as well as to perform targeted experiments to calibrate those models.
An open-acces paper describing the methodology is published in G-cubed:
Development of this software package was funded by the European Research Council under grant ERC CoG #771143 - MAGMA, and is currently supported by German Research Foundation (DFG) - Project number #521637679
Green, ECR, Holland, TJB, Powell, R, Weller, OM, & Riel, N (2025). Journal of Petrology, 66, doi: 10.1093/petrology/egae079
Weller, OM, Holland, TJB, Soderman, CR, Green, ECR, Powell, R, Beard, CD & Riel, N (2024). New Thermodynamic Models for Anhydrous Alkaline-Silicate Magmatic Systems. Journal of Petrology, 65, doi: 10.1093/petrology/egae098
Holland, TJB, Green, ECR & Powell, R (2022). A thermodynamic modelfor feldspars in KAlSi3O8-NaAlSi3O8-CaAl2Si2O8 for mineral equilibrium calculations. Journal of Metamorphic Geology, 40, 587-600, doi: 10.1111/jmg.12639
Tomlinson, EL & Holland, TJB (2021). A Thermodynamic Model for the Subsolidus Evolution and Melting of Peridotite. Journal of Petrology,62, doi: 10.1093/petrology/egab012
Holland, TJB, Green, ECR & Powell, R (2018). Melting of Peridotitesthrough to Granites: A Simple Thermodynamic Model in the System KNCFMASHTOCr. Journal of Petrology, 59, 881-900, doi: 10.1093/petrology/egy048
Green, ECR, White, RW, Diener, JFA, Powell, R, Holland, TJB & Palin, RM (2016). Activity-composition relations for the calculationof partial melting equilibria in metabasic rocks. Journal of Metamorphic Geology, 34, 845-869, doi: 10.1111/jmg12211
White, RW, Powell, R, Holland, TJB, Johnson, TE & Green, ECR (2014). New mineral activity-composition relations for thermodynamic calculations in metapelitic systems. Journal of Metamorphic Geology, 32, 261-286, doi: 10.1111/jmg.12071
Holland, TJB & Powell, RW (2011). An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. Journal of Metamorphic Geology, 29, 333-383, doi: 10.1111/j.1525-1314.2010.00923.x