Problem definition
Finding the most stable phase assemblage is a challenging constrained optimization problem including both equality and inequality constraints. One has to minimize the Gibbs energy (1) of the system while satisfying the Gibbs-Duhem (2) and mass equality constraints (3) while satisfying the mixing-in-sites inequality constraints (4).
1. Total Gibbs energy
The Gibbs energy of a multi-component multiphase system is given by the weighted summation of the chemical potentials of all end-members and pure phases:
where is the molar fraction of the solution phase,
is the molar fraction of the endmembers and
is the molar fraction of the pure phase.
The chemical potential of a phase is either a constant for a condensed (pure) phase:
or a function for a phase within a solution:
where is the thermochemical activity related to the mole fraction and the activity coefficient by
For the case of ideal mixing between the end-members, the activity coefficient is unity. The mixing of a species dissolved in a condensed phase, however rarely behaves ideally and is typically a function of both temperature and composition (mixing-on-sites formulation).
2. Gibbs-Duhem constraint
The Gibbs-Duhem constraint is defined as
where is the chemical potential of pure component (oxide)
and
is the molar compositional of component
in end-member/pure phase
3. Mass constraint
The mass equality constraint is defined as
where is the molar composition of component
in end-member
, and
is the molar composition of component j in a pure phase
Minimization approach
The Gibbs minimization approach employed in MAGEMin combines discretization of the equations of state in composition space with linear programming and extends the mass constrained Gibbs-hyperplane rotation method to account for the mixing-on-sites that takes place in silicate mineral solid solutions. For an exhaustive description of the methodology see Riel et (submitted).
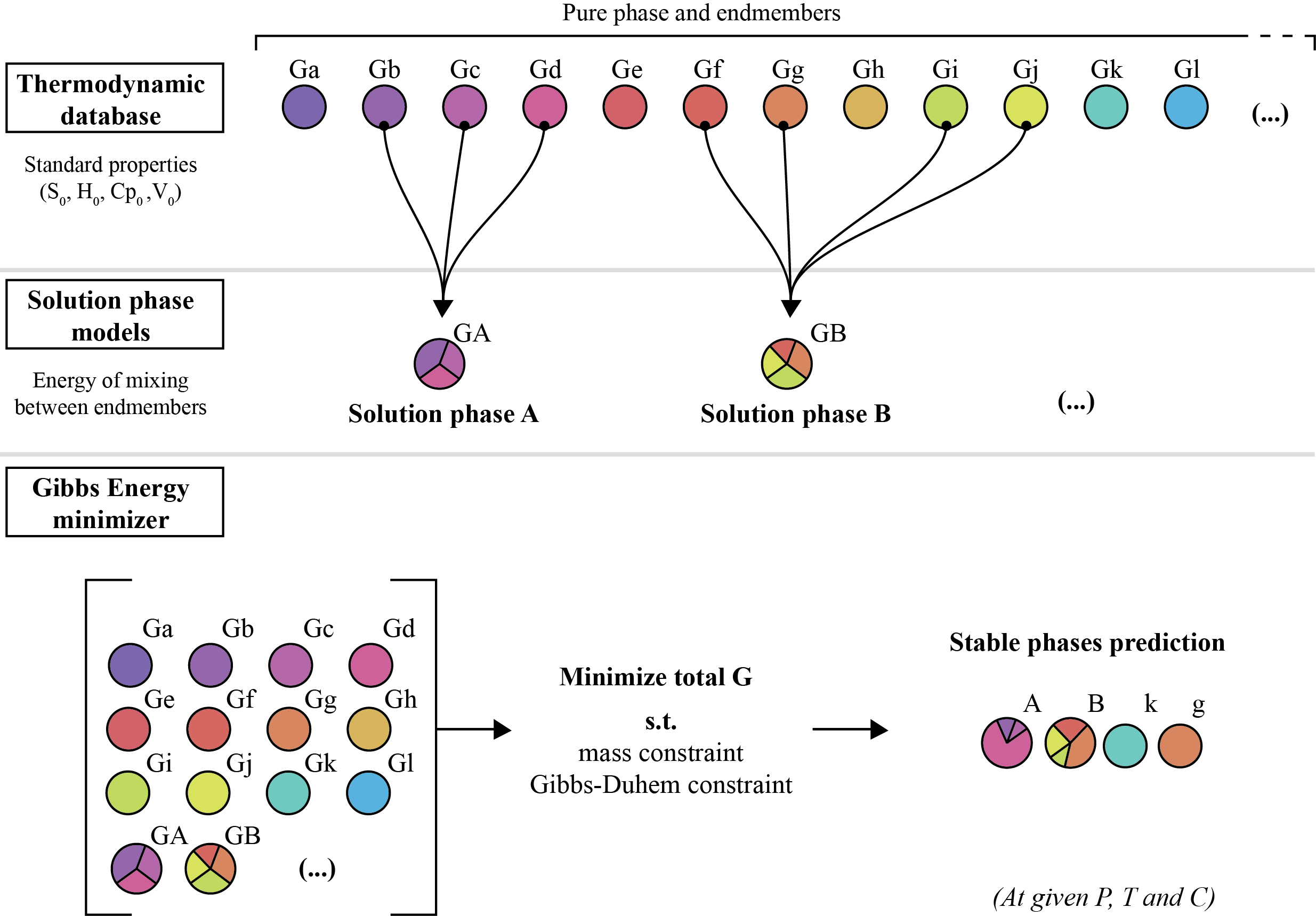
Algorithm demonstration
A simplified example of the Gibbs energy minimization approach used in MAGEMin is provided at:
https://github.com/ComputationalThermodynamics/SandBox/blob/main/MAGEMin_PGE_and_LP.m
This MATLAB application includes two pure phases, sillimanite and quartz, and activity–composition (a-x) relations for feldspar (pl4T, Holland et al., 2021) in a reduced Na2O-CaO-K2O-Al2O3-SiO2 (NCKAS) chemical system.
References
Holland, T. J. B., Green, E. C. R., & Powell, R.(2021). A thermodynamic model for feldspars in KAlSi3O8-NaAlSi3O8-CaAl2Si2O8 for mineral equilibrium calculations